The urine pH test is a painless, noninvasive test that measures the acidity or alkalinity of the urine sample.
The pH of your urine, whether acidic or alkaline, depends on your diet (for example, diets rich in meat are more acidic than others), medications, and diseases. Urine pH assessment is helpful in the evaluation of systemic acid-base disorder.

Urine is a rich body fluid with more than 3000 metabolites in it. Urinary components have the potential to diagnose numerous medical conditions—the urine composition changes according to age, food, race, gender, medications, and exercise.
Human urine is composed primarily of water, which is almost 95%. Others are urea (2%), creatinine (0.1%), uric acid (0.03%), phosphate, potassium, ammonium, sodium, chloride, sulfate, and other molecules.
Sometimes, a urine pH test is prescribed by your clinician if kidney stones are suspected. Finally, monitoring urine pH helps to assess crystalluria since some urinary crystals have the propensity to form in alkaline urine while others are in acidic urine.
What is a Urine pH test?
Urinary pH is an index of acid-base balance. It determines the acidity, alkalinity, or neutrality of a urine sample. Urine pH measures the hydrogen ion concentration in urine and is determined by the kidney’s ability to regulate hydrogen ion and bicarbonate concentrations within the blood.
Normal Urine pH
- The most common normal urine pH value of a sample that is freshly voided is 6 to 7.5 in individuals who are healthy and also note that any value between 4.5 to 7.8 is not much of a concern.
- Any value of pH more than 8 is alkaline or basic, and any pH less than 6 is acidic5.
Preparation for the urine pH test
Stop taking these medications before the test; these can affect its results. But don’t forget to consult the healthcare provider before quitting these medications.
- Thiazide diuretic
- Methenamine mandelate
- Sodium bicarbonate
- Potassium Citrate
- Acetazolamide
- Ammonium chloride
Procedure of the urine pH test
We can measure urine pH by using techniques like Nitrazine paper, Dipstick, Litmus paper, and Glass electrode. These techniques differ in their reading techniques and sensitivity.
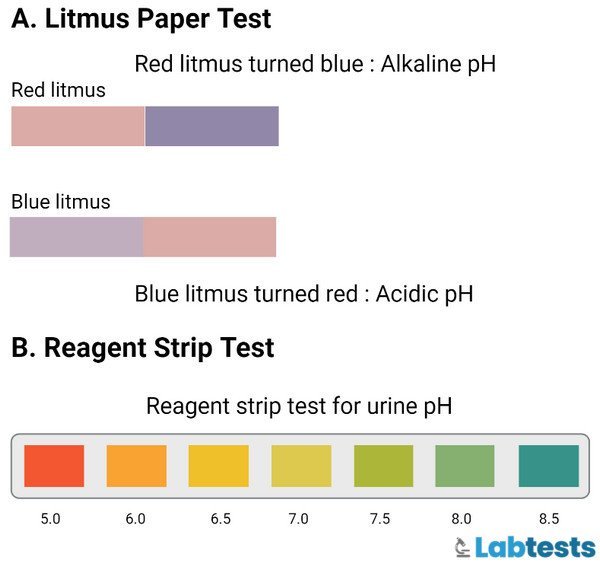
Litmus Paper
The litmus paper technique is a less sensitive method because it indicates only the alkalinity or acidity of urine and does not tell the exact quantity or figure of urine pH. This is a technique for pH measurement by using red and blue litmus paper. The procedure for the litmus paper technique is as follows:
- Collection of a freshly voided urine sample that should be well-mixed.
- Then tear small litmus paper of blue color.
- Dip the litmus paper into the urine sample and remove it immediately.
- Check for color changes. If the litmus paper’s color changes from blue to red, it shows that the urine is acidic.
- Now, tear small litmus paper of red color.
- Dip this red litmus paper into the urine sample and take it out immediately.
- And look for the color changes on the litmus paper red color. If the color of red litmus paper changes to blue, it shows that the urine is not acidic, it’s alkaline.
Nitrazine Paper
In this technique color changes from yellow to blue. Yellow is for acidic urine and blue is for alkaline urine. The chemical used to impregnate the paper is sodium dinitrophenol azo-naphthol disulfonate.
Change in color is due to the chemical. This technique matches the color change with the reference color chart. Depending upon the value of color change on the reference color chart, urine pH is recorded. The procedure for the Nitrazine Paper test is as follows:
The procedure of the Nitrazine Paper test is as follows:
- Tear small nitrazine paper.
- Then dip the nitrazine paper into freshly voided, and well-mixed urine sample and then remove the paper immediately.
- Use a reference color chart and compare the change in color.
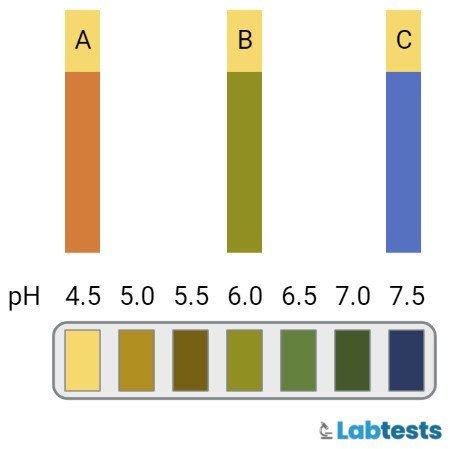
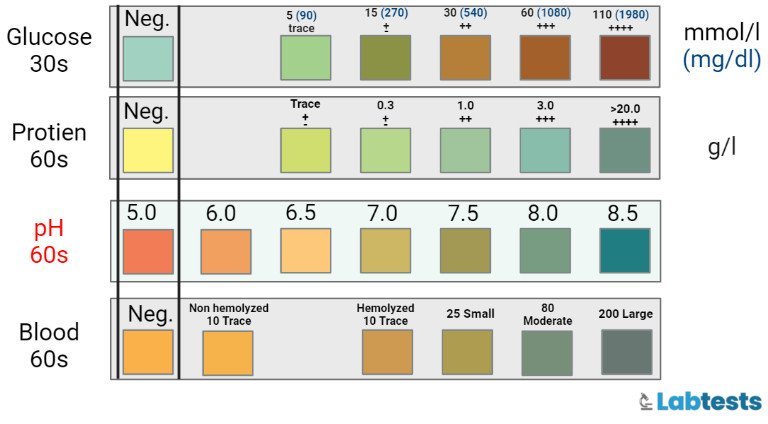
- The color change value should be recorded from the reference color chart that ranges from 3 to 4 to pH 9. pH of 3-4 is for yellow color and pH of 9 is for deep blue color. The result of urinary pH can be reported as acidic or alkaline.
Urine Dipstick Method
This reagent strip test was impregnated with bromothymol blue and methyl red chemicals. These chemicals depend upon hydrogen ion concentration in the urine and change their color from yellow to blue. Yellow color is for acid and blue is for alkali. The change in color is interpreted by its comparison with the reference color chart.
The procedure of the test is as follows:
- First, dip the reagent strip in a freshly voided, well-mixed urine sample and remove the reagent strip immediately.
- Tap the strip at the corner of the urine container and remove excess urine from the strip.
- Wait for full-color development and focus on the time that is mentioned by the manufacturer and then note the result.
- Then note the change in color and compare it with the reference color chart ranging from pH 5, which is for acidic urine to pH 9, which is for alkaline urine. Yellow color shows acidic and blue shows alkaline.
- Report the result.
Glass Electrode
It is a pH measurement technique that is very sensitive. A small electronic instrument with a glass electrode and an electronic pH value indicator is used for measurement. It operates on electricity or a battery.
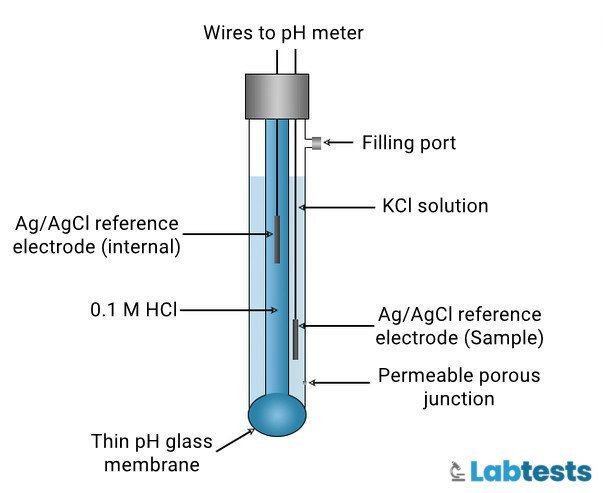
The procedure for this technique is to dip the glass rods in well-mixed and freshly voided urine samples. After that look for the figure in the instrument and find out the pH.
What is the Clinical Significance of the urine pH test?
The urine pH test is clinically significant. The renal system, including kidneys, ureters, urinary bladder, and other structures, is necessary to regulate blood pH, i.e., to keep it at 7.4 + 0.05. Urine pH is helpful to diagnose acid-base disorders. It is also used to identify the type of crystals.
Blood pH is maintained by the absorption or release of hydrogen ions. Suppose blood pH is acidic, i.e., below 7.35. In that case, nephrons absorb the hydrogen ions from surrounding blood vessels, and when it’s alkaline, i.e., above 7.45, the release of hydrogen ions to the surrounding blood supply.
Causes of Acidic and Alkaline urinary pH
| Acidic pH of urine (<6) | Alkaline pH of urine (>8) |
|---|---|
| Diabetic ketoacidosis | Anorexia nervosa |
| Starvation | Certain vegetables, citrus fruits, and milk products also may cause alkaline urine, which is not pathological. |
| Fever | Alkalizing medicines like streptomycin or kanamycin for UTI. |
| Dehydration | Vomiting |
| Diarrhea | UTI |
| Malabsorption syndromes | Renal failure |
| Certain drugs like Phenacetic | Alkalosis (metabolic or respiratory: due to accumulation of CO2 in the body) |
| High protein diets may also result in acidic urine, but this is not pathological. |
Significance of urine pH in renal stone patients
Urine pH measurement is also essential in managing patients with renal calculi and those on diets or medications to change the urine pH.
- Calcium phosphates, magnesium phosphate, and calcium carbonate renal stones develop in the urine when it’s alkaline. In such instances, the urine must be acidic (i.e., by diets such as meat or medications).
- Uric acid, calcium oxalate, and renal cystine stones are precipitated in the urine when it’s acidic. Therefore, the urine should be kept alkaline (i.e., by diets such as leguminous plants, citrus fruits, and most vegetables or by medications).
What are the causes for decreased urine pH?
The common causes of acidic urine pH or decreased urine pH are
- Metabolic acidosis due to Diabetic ketoacidosis (DKA), ethylene glycol intoxication, Renal failure, Addison’s disease, and Lactic acidosis,
- Paradoxical aciduria defined as acid urine pH with metabolic alkalosis is also a cause and it is due to severe vomiting
- High protein or milk-based diet
Some less common or uncommon Causes are
- Medications like Salicylate, Carbonic anhydrase inhibitors, Methanol, Furosemide, Urinary acidifiers, and Paraldehyde,
- Vomiting with chloride depletion
- Fever, starvation, or prolonged exercise
- Hydrogen production by bacteria
- Severe diarrhea
- Respiratory acidosis
- Decreased serum potassium
- Proximal renal tubular acidosis with depletion of bicarbonate
What are the causes of increased urine pH?
The common causes of alkaline urine pH or increased urine pH are
- Urinary tract infections with organisms like urease-producing bacteria for example Staphylococcus spp and Proteus spp.
- Artifacts for example
- delayed sample analysis, that causes spontaneous degeneration of urea
- Pigmenturia
- The recent meal, also known as postprandial alkaline tide
Some less common or uncommon Causes are
- Proximal renal tubular acidosis
- Distal renal tubular acidosis
- Respiratory alkalosis
- Diets rich in vegetables and cereals
- Metabolic alkalosis
Frequently asked questions
Q1. What does it mean if urine pH is less than 7?
A urine pH of 7 indicates neutrality, and a pH of < 7 shows acidity.
Q2. What does it mean if urine pH is more than 8?
A urine pH of 7 indicates neutrality, and a pH > 8 indicates alkalinity.
Q3. Which specimen is used for the urine pH test?
Urine is a specimen type to be used for urine pH tests. Around 10 mL urine sample is required that is collected randomly in a 10 mL urine collector or container.
Q4. What is the false interpretation of urine pH test?
When the urine specimen is left for more than 2 hours, microorganisms like bacteria will grow in it. Urinary pH will become alkaline due to the conversion of urea into ammonia. The result is false alkaline and it indicates that the specimen was not fresh.
References
- Tietz Textbook of Clinical Chemistry and Molecular Diagnostics – 6th Edition. (2017, January 16). Retrieved October 21, 2022, from https://www.elsevier.com/books/tietz-textbook-of-clinical-chemistry-and-molecular-diagnostics/rifai/978-0-323-35921-4
- Worcester EM, Bergsland KJ, Gillen DL, Coe FL. Mechanism for higher urine pH in normal women compared with men. Am J Physiol Renal Physiol. 2018 Apr 1;314(4):F623-F629. doi: 10.1152/ajprenal.00494.2017. Epub 2017 Dec 20. PMID: 29357436; PMCID: PMC5966764.
- Urine pH. (2020, October 7). ucsfhealth.org. Retrieved October 21, 2022, from https://www.ucsfhealth.org/medical-tests/urine-ph-test
- What to Know About a Urine pH Test. (2021, June 25). WebMD. https://www.webmd.com/a-to-z-guides/what-to-know-about-a-urine-ph-test
- American Association for Clinical Chemistry: “Urinalysis.”